Bio-Technology, Cleanroom News, FDA
Contamination Control News: Examining the Scope of the Problem.
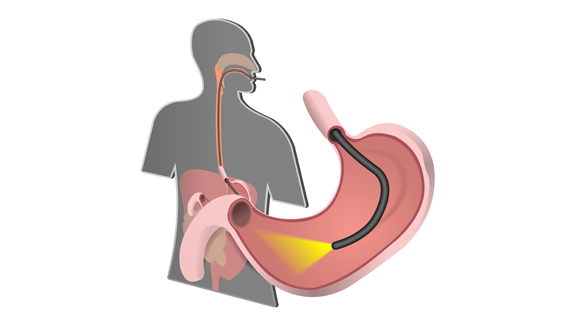
Imagine the scenario: after experiencing weeks of sudden and unexplained pain in your stomach, your doctor – suspecting ulcers – refers you to the hospital for tests. Or perhaps you rush your toddler to the ER after he swallowed part of his favorite action figure. In either of these cases, your immediate future involves getting uncomfortably familiar with the business end of a duodenoscope – a flexible lighted tube that snakes its way through the mouth and stomach until it reaches the top of the small intestine – the duodenum. Once in position, it offers the least invasive way of pinpointing that ulcer or of locating Batman’s head.
And in either of these situations your very last concern is whether the scope that’s currently circulating just south of your esophagus en route to your stomach is clean. You’re in a hospital, right? Of course it’s sterile. But that is where you might be wrong. And the results could be deadly.
Letters from America
In 2013 and 2014, hospitals in Europe received two warning letters from Olympus Corporation (aka Olympus Corporation of the Americas) head-quartered in Pennsylvania. Olympus is the manufacturer of the eponymous Olympus Video Duodenoscope TJF Q180V and the warnings were intended to highlight a horrifying danger presented by this device.(1) The first of these letters, termed an ‘Important Safety Notice,’ included a detailed reference guide to the correct pre-cleaning and reprocessing of each scope. But why the sudden need for an updated reference guide? The truth makes for grim reading…
In January 2013, sufficient data had been acquired for the manufacturers to be concerned that the cleaning processes normally mandated for these devices were inadequate. The complexity of the device coupled with its functional demands meant that the existing SOPs for inter-patient sterilization were simply not good enough. Indeed in the follow-up warning, the company urged ‘field safety corrective action’ due to complaints of ‘residual debris in the distal end of the [scope].’
Residual debris in the distal end? Let’s take a moment to examine that phrase.
In non-medical terms, this sanitized language means to convey the fact that contaminants – blood and tissue – from one patient remained trapped in the head of the device, even after cleaning and before use in the next patient. And given where that scope had been, this was clearly a cause for some concern.
Under US law, it is incumbent upon medical device manufacturers to report incidents like these to the FDA within thirty days of occurrence.(2) Yet despite the prevalence not only of these devices but also of this specific model in US hospitals, no report was filed to warn of the grave risk of cross-contamination. In fact, between 2012 and 2014 as alerts were issued to medical practitioners in Europe, patients in eight US hospitals suffered infections traced back to the use of tainted duodenoscopes. At two care facilities in Los Angeles alone – UCLA’s Ronald Regan Medical Center and Cedars-Sinai Medical Center – the scopes were indicated as vectors for the transmission of antibiotic-resistant superbug which caused devastating illnesses and death.(3) And the much publicized outbreaks in California and Washington were only the beginning.
The Big Picture
A day after news broke on the UCLA deaths, Olympus Corp issued an initial alert to the FDA – almost two years after flags were raised in the European community. An extensive inquiry by Representative Ted Lieu (D-Torrance) along with staff from the House Oversight and Government Reform committee followed and slowly the true scope of the problem emerged, with the committee’s report painting a decidedly unpleasant picture of superbugs and deadly infections.
Although affected medical facilities and responsible device manufacturers cannot, under federal law, be specifically named by the FDA, reports show that between January 2010 and October 2015 an estimated 350 to 404 patients at 30 facilities across the nation (and a further 11 facilities overseas) were infected by contaminated scopes.
‘Superbug’ is an evocative term beloved of the media. Conferring an albeit malicious super-status, it conjures up an apocalyptic scenario of out-of-control bacterial infections running rampant, slaying the unsuspecting and leaving devastation in their wake. Consider them, if you will, the Kardashians of the contamination-control world – they’re everywhere and, however hard you try, you just can’t get rid of them. And that dramatized image is not too far from the truth. According to the CDC, approximately two million patients are sickened annually by superbugs – bacteria resistant to multiple forms of antibiotics – and about twenty-three thousand of these people will die.(4) Carbapenem-Resistant Enterobacteriacaea (CRE) is an example of a superbug (E. coli being a prominent member of that family) as is Neisseria gonorrhoeae, the bacterium that causes gonorrhea. With a CRE kill rate of 50%, clearly these are not bacteria to which we should open the doors of our centers of healing…
The White Knight of Legislation
Fortunately, governmental oversight may yet come to our aid. The responsibility device manufacturers have to their European customers may soon be extended to US medical practitioners. Rep. Lieu has filed a bill, termed not surprisingly the Device Act, that would require companies such as Olympus to notify the FDA when they update the design of, or cleaning SOPs for, such medical devices. And additionally, enhanced validation will be required to ensure that cleaning SOPs deliver what they promise – contamination control.
As of publication date, despite a voluntary recall, problematic scopes remain in use in hospitals while the manufacturer works on a design solution aimed at reducing the contamination risk. And it is now incumbent upon hospitals to test their scopes after cleaning and during a recommended 48-hour quarantine period during which any bacterial growth is assessed. Clearly this is not an adequate response to a grave risk to our health and we are interested to see how to story develops over time.
Please let us know your thoughts too – simply comment below!
(1) Olympus Warned Europe about Superbug Scope Dangers in 2013
(2) Bacterial Infections Associated with Duodenoscopes: FDA’s Actions to Better Understand the Problem and What Can be Done to Mitigate It
(3) Number of deadly infections from dirty scopes is far higher than previously estimated
(4) Superbugs: What They Are and How You Get Them

















Hello there! I could have sworn I’ve been to this blog before but after reading through some of the post I realized it’s new to me. Anyways, I’m definitely glad I found it and I’ll be book-marking and checking back frequently!